Investigating the role of regulatory T cells (Tregs) in modulating immune responses to promote lung transplant acceptance
ALI scores and images of MHC class II+ cells and Tregs in grafts
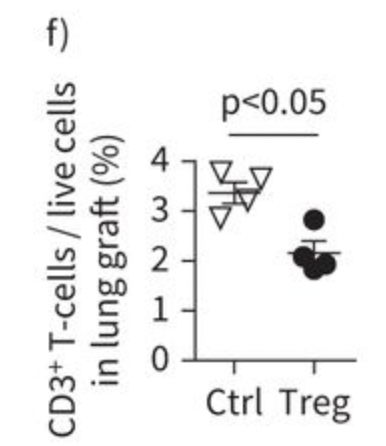
Caption
Our laboratory investigates novel approaches to improve lung transplant outcomes through the manipulation of regulatory T cells (Tregs). In groundbreaking research, we demonstrated that recipient-derived Tregs can be successfully delivered to donor lungs during ex vivo lung perfusion (EVLP) prior to transplantation. Using a rat model of EVLP followed by lung transplantation, we showed that these Tregs maintain their immunoregulatory function and can effectively enter the lung tissue without causing damage. Our work revealed that pre-transplant Treg delivery creates an immunoregulatory environment within the donor lung, leading to reduced activation of inflammatory T cells in the early post-transplant period. This innovative approach of organ-directed cellular therapy represents a significant advance in the field, as it allows us to establish immune regulation within the allograft before transplantation occurs. Our findings suggest that this strategy could potentially prevent rejection and improve long-term outcomes in lung transplant recipients. We are continuing this line of research in a novel mouse model of pre-transplant lung allograft-directed Treg cell therapy, and we continue to explore other novel means to leverage Tregs to promote transplant tolerance and prevent chronic rejection.
Publication:
Miyamoto E, Takahagi A, Ohsumi A, Martinu T, Hwang D, Boonstra KM, Joe B, Umana JM, Bei KF, Vosoughi D, Liu M, Cypel M, Keshavjee S, Juvet SC. Ex vivo delivery of regulatory T-cells for control of alloimmune priming in the donor lung. Eur Respir J. 2022 Apr 14;59(4):2100798. doi: 10.1183/13993003.00798-2021. PMID: 34475226.
Developing and utilizing mouse lung transplant models to study the cellular and molecular drivers of rejection
The Juvet lab devised two novel innate immune-augmented mouse lung transplant models of CLAD that recapitulate the histopathology seen in the restrictive and obstructive types of human CLAD – restrictive allograft syndrome (RAS) and bronchiolitis obliterans syndrome (BOS), respectively. We are now using these models to uncover the complex cellular and molecular mechanisms driving transplant rejection. Through these models, we have made several key discoveries about the pathways leading to chronic lung allograft dysfunction (CLAD). In one significant study, we demonstrated that ischemia-reperfusion injury promotes B cell-mediated RAS-like chronic rejection through the formation of tertiary lymphoid organs within the allograft and the production of autoantibodies. This work revealed that B cell depletion can attenuate both acute and chronic rejection, highlighting B cells as potential therapeutic targets. In complementary research, we investigated the role of classical dendritic cells (cDCs) in lung transplant rejection using Batf3-deficient mice. We discovered that donor and recipient cDCs play distinct and context-dependent roles in rejection: while recipient Batf3-dependent cDCs promote rejection during airway inflammation that leads to BOS-type pathology, donor Batf3 expression helps prevent rejection under baseline conditions. Recent collaborative work with the Martinu lab shows that mouse lungs in our BOS and RAS models share many gene expression patterns seen in human BOS and RAS lung tissue. These findings demonstrate how our mouse models enable us to dissect the complex cellular interactions that occur during rejection and identify novel therapeutic targets. Through this work, we continue to advance our understanding of the immunological mechanisms that lead to CLAD and develop new strategies to prevent rejection and improve transplant outcomes.
Publication:
Watanabe T, Lam C, Oliver J, Oishi H, Teskey G, Beber S, Boonstra K, Mauricio Umaña J, Buhari H, Joe B, Guan Z, Horie M, Keshavjee S, Martinu T, Juvet SC. Donor Batf3 inhibits murine lung allograft rejection and airway fibrosis. Mucosal Immunol. 2023 Apr;16(2):104-120. doi: 10.1016/j.mucimm.2023.02.004. Epub 2023 Feb 25. PMID: 36842540.
Watanabe T, Martinu T, Chruscinski A, Boonstra K, Joe B, Horie M, Guan Z, Bei KF, Hwang DM, Liu M, Keshavjee S, Juvet SC. A B cell-dependent pathway drives chronic lung allograft rejection after ischemia-reperfusion injury in mice. Am J Transplant. 2019 Dec;19(12):3377-3389. doi: 10.1111/ajt.15550. Epub 2019 Aug 26. PMID: 31365766.